
Latest page content update: 1 Jul 2024
Introduction
CDISC Conformance Rules are an integral part of the Foundational Standards and serve as the specific guidance to Industry for the correct implementation of the Standards in clinical studies. An emerging Industry best practice is to use Conformance Rules on an ongoing basis, throughout the study, to keep the data as close to submission ready as possible and to ensure quality in all data exchange scenarios.
Current CDISC Conformance Rules need to be expressed in a common specification to be loaded to the CDISC Library. In addition, an executable component must be developed for every Conformance Rule.
Project Goals and Objectives
The overall goal of the CORE Project is to deliver a governed set of unambiguous and executable Conformance Rules for each Foundational Standard, and to provide a Reference Implementation of an open-source execution engine for the executable Rules.
The global clinical research community will be able to leverage the free and open CORE software to test study data for conformance to CDISC standards as well as to regulatory and sponsor-specific conformance rule sets.
The CORE Project objectives are to:
- Ensure each standard has a set of unambiguous, executable Conformance Rules
- Ensure consistency across Conformance Rule implementations
- Expedite the availability of executable Conformance Rules for new Foundational Standards
- Create executable Conformance Rules vetted by the CDISC standards development teams
- Create a Reference Implementation of an open-source engine that executes the Rules
- Release the open-source engine under the CDISC Open-Source Alliance (COSA)
Further Considerations
- The CORE project includes development of:
- Executable Conformance Rules for the CDISC standards
- Executable Conformance Rules for FDA Business Rules
- A Reference Implementation of a software engine (CORE) to execute these rules
- CDISC is publishing the executable Conformance Rules in the CDISC Library
- CDISC provides free access to CORE to CDISC members and non-members
- CORE is published as open-source (MIT license)
- CDISC has no plans to deploy CORE as commercial software
- Developers have the option to prepare CORE for:
- On-premises deployment
- A cloud-platform deployment
- Running from command line, integrating with other systems
- Implementers may choose to develop a proprietary engine or adapt CORE
- The CORE Engine Reference Implementation can confirm that a proprietary engine achieves the correct results
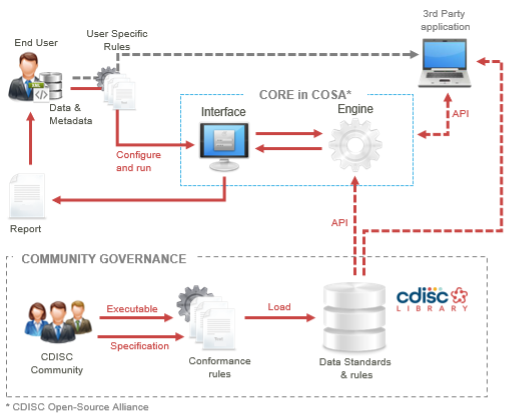
Project Concept Diagram
The following diagram illustrates the concept of the CORE project, including the Conformance Rules, the executable form of the Rules, and the Rules execution engine:
CORE Program Roadmap

How to Participate
We invite your organization to participate in this exciting new project.
Please visit the CORE Project Participate tab to learn more.
Additional Resources
CORE Charter
CDISC Announces Research Collaboration with FDA to Incorporate FDA Business Rules into CORE
Latest page content update: 1 Jul 2024
CORE Next Steps
- Work toward completing the executable Conformance Rules for the Foundational Standards as well as the Regulatory business rule sets, targeting a complete set of Conformance.
- Update the CORE Engine and Rule Editor with the necessary new functionality as new Conformance Rule requirements emerge.
- Implement a formal governance process for Conformance Rules.
- Continue dialog with software vendors considering development of CORE offerings.
- Implement a certification program for proprietary Rule execution engines.
- Provide a validation package for CORE that implementers can extend to offer a fully validated solution.
Milestones
Summer 2024:
SGS' Roman Radelicki captivated COSA Spotlight attendees with his presentation 'CORE-Authoring and Running Your Own Rules', emphasizing the versatile application of Conformance Rules not just as submission standards, but as essential tools throughout the clinical data lifecycle. We strongly recommend you watch it and see how the syntax and engine of these rules can seamlessly facilitate communication and collaboration among stakeholders.
Start 2024:
- Research collaboration agreement signed with FDA for the creation of FDA Business Rules in the CORE syntax.
- Ongoing discussion with software vendors and pharma/biotech for the implementation of the CORE engine in their own systems..
- First attempts for the creation of company-specific rules in the CORE syntax.
Volunteers are needed for Conformance Rule development. To sign up, please visit the “Participate” tab.
Spring 2023:
- The first free, publicly available CORE Engine desktop deployment by an industry software vendor (Formedix) is now available!
- Formedix uses a technology preview version of the CORE Engine to which it has added its own user interface. This simple-to-install and use desktop version makes it easier for users to evaluate the CORE Engine and Rules without IT support. Formedix discusses this CORE offering in a blog post.
- The public can download the offering via the Formedix website here. The download package includes test data publicly available from CDISC to jumpstart use; user study data can also be referenced. This Formedix CORE offering accesses all the CORE Rules currently available in the CDISC Library (at time of release).
The uptake of CORE by the CDISC community is already under way! Several software providers have demonstrated the use of CDISC CORE in their tools to drive adoption.
Fall 2022:
- Initiated Conformance Rules development for remaining CDISC Foundational Standards
- Initiated ongoing dialog with FDA about eventual CORE Rules adoption.
- Discussed early provision of the CORE Engine with industry software vendors, with focus on desktop versions that are easy to deploy and feature a user interface of the vendor’s design. These simple to install and use desktop versions will make it easier for the CDISC community to evaluate the CORE Engine and executable Rules and provide valuable feedback to CDISC.
Summer 2022:
- Transitioned the Engine and Rule Editor development from a traditional CDISC project to open-source: (1) released under the MIT license; (2) registered with the CDISC Open-Source Alliance (COSA); (3) published on the project’s public GitHub repository; (4) provided with a command line interface.
- Established the CORE Roadmap Board, composed of industry representatives and CDISC leadership, to expand industry input as Conformance Rule development and open-source software development continued.
- Enhanced Rule Editor training for all volunteers.
2022 CDISC Europe Interchange, April 2022:
- The first deployment of the MVP CORE Engine, a minimum-featured evaluation version in the CDISC cloud and free to all, was announced and demonstrated.
- About two-thirds of the 336 executable rules in SDTMIG 3.4 were published in the CDISC Library and accessible via Library API as well as to the CORE Engine and other software tools.
Summer and Fall 2021:
- Development began on a minimum viable product (MVP) Rule Editor and execution Engine.
- Conformance Rule authoring began as the earliest versions of the Rule Editor and Engine became available. The initial rule authoring focus was on Rules for the SDTMIG 3.4.
Latest page content update: 24 Nov 2022
Project milestone reached: 28 April 2022
Evaluation version of CORE Engine Reference implementation was deployed on CDISC’s Azure cloud subscription:
- Available to users for free
- Initially includes 200+ Rules for SDTMIG v3.4
- Test data is provided by CDISC; users cannot provide their own data
- Visit the TRY IT NOW tab to access this deployment
Project milestone reached: 31 August 2022
Transition of the CORE Engine Reference Implementation to the open-source environment was completed with provision of the Engine in GitHub. The Engine is:
- Provided as open-source with the permissive MIT license
- Registered with the CDISC Open-Source Alliance (COSA)
- Available to users for free
- Provided with a command line interface (CLI)
- Accessed at the GitHub CDISC-rules-engine repository, including special instructions in the Readme file
CORE Rules Development
Rules Planning
- An inventory of Rules per Foundational Standard has been developed and is presented in the following graphic:
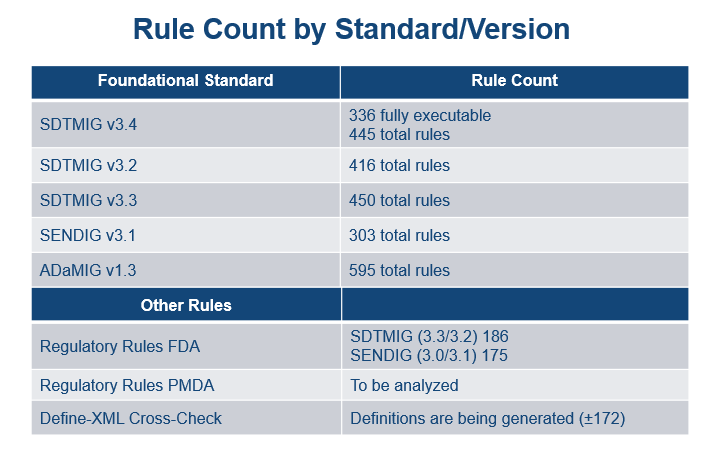
- A timeline for development for these Rules has been prepared and is presented in the following graphic. Note that much of the work for SDTM 3.4 has been completed already.
- The remaining work represented in the timeline is estimated to require about 3.0 FTE for about 8 months, followed by about 1.0 FTE for an additional 6 months. This timeline assumes this level of resourcing will become available to the project.

Rules Status
- The work on the SDTM, SEND, and Define Cross-Checks has recently begun with the limited number of volunteers currently available.
- The CORE project is actively recruiting additional volunteers for this work right now. (See the CORE Roadmap Board activities below on this web page (tab) for more about this).
- A “CORE Volunteer Onboarding Training” webinar for this Rules development was held June 7, 2022. The webinar covered the scope of Rules, the Rule Editor for authoring and testing Rules, and how to sign up and participate. Listen to the CORE Volunteer Onboarding Training. We invite you to visit the Participate tab to sign up to participate on CORE.
- Beyond the webinar, other on-boarding materials to support Volunteers have been delivered:
- Training materials
- Quarterly training sessions to onboard new rule authors
- Rules development workshops at:
- 2022 CDISC US Interchange
- 2022 PHUSE EU Connect
CORE Engine Development
Engine and Deployments Planning and Status
- The evaluation version of the CORE Engine Reference Implementation was previously deployed to the CDISC cloud subscription (April 2022) and to the Azure Marketplace (June 2022).
- Following these deployments, and as part of the transition of CORE from a traditional CDISC project to an open-source framework, CDISC has released the Engine on GitHub.
- Going forward, the open-source Engine development community will be responsible for future Builds of the Engine. These Builds will continue to improve functionality and features of the Engine, as needed. The team anticipates multiple Beta releases with less frequent Stable releases made available. All Engine Builds will be made available on GitHub.
- Engine deployments will be the responsibility of the users. This will include end-users such as Pharma-Biotech and CRO organizations as well as commercial software vendors and integrators deploying for end-users.
- Currently, several vendors have confirmed they will publish free desktop deployments. More communication is expected to follow.
- In addition, the CORE project anticipates that a small number of end-users will integrate the Reference Implementation into their environment during 2023.
- The following graphic illustrates this planning:

Latest page content update: 24 Nov 2022
Open-Source Governance and Development
During the summer of 2022, after completion of the initial set of executable Conformance Rules (for SDTM 3.4) and release of the evaluation version of the CORE Engine Reference Implementation, CDISC transitioned the CORE project from a traditional CDISC-led project to an open-source framework. The transition focuses on:
- Establishment of a CORE Roadmap Board and CORE Technical Committee to guide and coordinate further Engine Reference Implementation development as open-source and to coordinate with CDISC on CDISC’s further Conformance Rules development.
- Establishment of the CORE Engine Reference Implementation software as open-source by making it publicly available in GitHub and registering it with COSA (CDISC Open-source Software Alliance). Further Engine Reference Implementation development will be conducted under the MIT open-source license by a volunteer, open-source development team.
The following graphic illustrates the full open-source framework for further CORE development. The framework centers on the CORE Roadmap Board and CORE Technical Committee and involves CDISC staff, industry volunteers, and commercial software vendors.
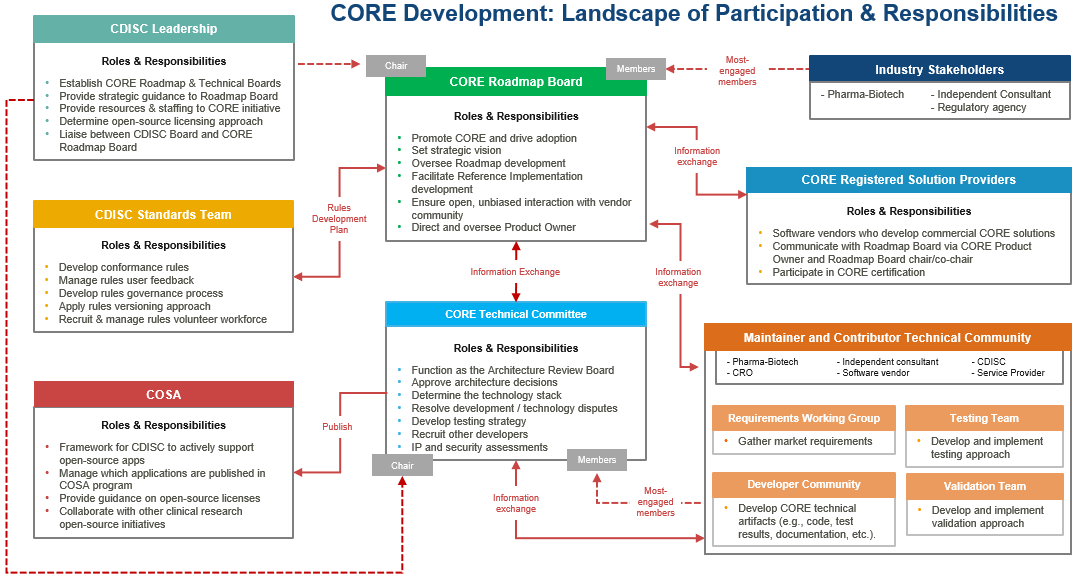
CORE Roadmap Board
The Board is composed of most-engaged CORE stakeholders from Pharma-Biotech, CDISC, Independent consultants, and Service providers. Engaged software vendors have a parallel opportunity to participate today on the CORE Technical Committee and a future opportunity to participate in the CORE Registered Solution Providers program which will be established by the Board.
Following are high-level highlights of the CORE Roadmap Board’s responsibilities:
- Promote CORE and drive adoption
- Set strategic vision
- Oversee Roadmap development
- Facilitate Engine Reference Implementation development
- Ensure open, unbiased interaction with vendor community
Following are the CORE Roadmap Board members:
| Industry | CDISC |
|---|---|
| Anne-Sophie Bekx, Janssen | Dave Evans, CEO |
| Chris Decker, Instem | Sam Hume, VP Data Science |
| Praveen Garg, Astra Zeneca | Peter Van Reusel, CSO |
| Brooke Hinkson, Merck | |
| Stephen Matteson, Pfizer | |
| Girish Rajeev, Takeda |
Board meetings are currently ongoing approximately monthly.
CORE Technical Committee
The Technical Committee is comprised of the most-engaged CORE stakeholders from the CORE Maintainer and Contributor Technical Community. These are the volunteers who are currently most active in the technical side of development of the CORE Engine Reference Implementation.
Following are high-level highlights of the CORE Technical Committee’s responsibilities:
- Function as the Architecture Review Board
- Approve architecture decisions
- Determine the technology stack
- Resolve development / technology disputes
- Develop testing strategy
- Recruit other developers
- IP and security assessments
Latest page content update: 17 Jan 2024
The CORE Project seeks volunteers for our Conformance Rules Development Team
- Mission:
- Develop the rules specification and the executable form of the rules.
- Develop the rules specification and the executable form of the rules.
- Work Areas:
- Specification structure and content
- Executable rules content
- Rule Developer Skill Set
- Core Skills
- Data savvy with science background; e.g., statistics, biometrics, data science
- A CDISC standards practitioner. Solid implementation experience with SEND, SDTM, and/or ADaM
- Experience in data specifications & associated verification & validation tasks
- “Plus” Skills
- Some familiarity with the associated conformance rules
- Knowledgeable in structured data, such as XML, JSON, YAML
- A member of an organizational standards council or governance body
- People without coding experience can succeed in rule development. The carefully prepared initial training program and ongoing support process enable this.
- Core Skills
Expected Engagement
- Time Period: 3 - 6 months commitment, or longer if able
- Hours per week: efforts such as 20%, 40% FTE will be helpful
- Weekly Meetings and Workshops: Recorded and available for review
CORE Volunteer Onboarding Training Webinar
A CORE Volunteer Onboarding Training webinar will be scheduled for early February. The webinar will cover the scope of Rules, the Rule Editor for authoring. and testing Rules. Listen to the CORE Volunteer Onboarding Training.
Sign-up Instructions
- If you would like participate in this exciting effort, sign up on the CDISC volunteer page and select CORE Rules on the form as the Standards Development team. Please include which CORE team you would like to join in the box at the bottom of the page.
CDISC held a webinar 20 July 2021 to provide a deeper understanding of the CORE project, share the “ask” of participants and answer questions from attendees. We invite you to listen to the webinar recording to learn more.
The following are links to CORE presentations from the 2023 CDISC US Interchange and PHUSE EU Connect, respectively:
- Title: Conformance Rules CDISC Open Rules Engine
- Presented By: Peter Van Reusel, Chief Standards Officer, CDISC and Amy Palmer, Head of Standards Operations, CDISC
- Date: 18 October 2023
- Title: CDISC Conformance rules and the CDISC Open Rules Engine Continuing the Road to Adoption
- Presenter: Nick De Donder, CORE Product Owner, CDISC
- Date: 6 November 2023
Article:
The CDISC Open Rules Engine: Open-Source Software for Clinical, an article in Clinical Leader by Sam Hume, VP, Data Science, CDISC
Latest page content update: 7 Mar 2024
Library Access
Please visit the Access tab to set up an account.
- Before CORE was deployed, CDISC has implemented a version of the Library accessible to non-members to ensure that the access needed to use CORE is available to all.
- A CDISC Library API key, accessible through a Library account, will be required for CORE to retrieve executable rules and standards.
Data Format
- CORE will ingests a variety of dataset formats, including SAS v5 XPORT, Dataset-JSON, and CSV.
- As an open-source application, vendors can extend CORE to add support for additional dataset formats.
Executable Rules Language and Rules Editor
All executable rules for CORE (“Rules”) are expressed in YAML. YAML is a non-proprietary data interchange language. Rules are authored in the Rule Editor, which is an on-line web application. Prior knowledge of any programming languages is not required to author Rules in the Rule Editor. All Rules are based on one data model, from which we deduce a schema. This schema is the backbone of the Rule Editor, providing syntax hints and validations.
CDISC maintains rule sets along with publication of foundational standards. Many conformance rules in these rule sets can easily be transcribed into metadata and rule logic, whose structure is bound to the schema. Schema validated Rules are sent to the CORE Rule Engine (“Engine”) for execution.
The Engine provides a framework to plug-in new rule types developed by the CORE team or by other implementers that have the need for additional types of rules.
CDISC plans to only test and validate the Rules with the Engine; however, vendors and sponsors will have the option to develop their own applications to work with the Rules, under an open-source arrangement.
Format Reports
- CORE reports will be produced in Excel to facilitate day-to-day operations by CORE users. JSON outputs are also available.
- CORE plans to support SDRG-XML so that the output of the rule execution will be available in a reviewer’s guide format.
- Other technical outputs such as XML, JSON, and CSV are planned for later CORE versions or update release.
- Vendors and sponsors will have the ability to extend the open-source CORE engine to provide reports in additional output formats.
Integration
- The executable Conformance Rules will be available in the CDISC Library, and a rich set of APIs will be provided for the CORE engine to access these rules.
- The open-source CORE engine can be integrated into or run by existing clinical data systems to add a standards conformance capability.
- The CORE system will also provide an API to enable integration with existing clinical data systems.
- In other words, users can create hard endpoint integrations or API endpoints for the CORE engine with their existing systems.
- CDISC plans to create implementation documentation, more information can already be found in the README on GitHub.
- CDISC also plans to create a dedicated LinkedIn channel for users to share their experience and integration stories.
Relationship to Commercial Systems
- The CORE engine will be open-source, available to all. It will not be offered by CDISC as a commercial product.
- The CORE engine will be designed to execute the CDISC Conformance Rule sets.
- The CDISC Conformance Rules will be an integral part of the CDISC Standards; they will be developed by CDISC with the Community and will be governed by CDISC standards development processes.
- The CDISC Conformance Rules will be an unambiguous, executable set of rules for CDISC Standards.
- The Conformance Rules for new CDISC Standards will be developed and released in a timely manner (i.e., as the new standards are released).
- The CDISC Conformance Rules will be a single source of truth, open-source and available to all in the CDISC Library.
- The CDISC Library will provide a rich set of APIs to access the CDISC Conformance Rules.
- The CDISC Conformance Rules will be expressed in a common/layman language.
- Vendors and sponsors will be able to integrate the open-source CORE engine into their operational systems.
Regulatory Agency
- CDISC works with Regulatory Agencies to ensure input for the conformance rules is compliant with published requirements for each Regulatory Agency. Regulatory Agencies continue to participate in these discussions.
- Yes, CDISC expects to include Regulatory Agency-specific rules within its full set of rules in CORE.
- It is expected that the user can run this specific rule set on the data.
- There are small but important implementation differences between the CDISC Conformance Rules and the Regulatory Agency-specific rules.
- While CDISC continues to work with the Regulatory Agencies to minimize implementation differences, CDISC expects that the user will be able to minimize any differences by checking conformance against multiple rulesets Further, the user will be able to configure the rule sets to meet their needs.
Access to CORE Source Code via GitHub
The CORE source code has been released as open-source on GitHub.
Yes, CDISC will provide training on GitHub, rules creation and running the rules with the CLI.
CORE API
Yes, CORE has a REST API.
Validation of a CORE Implementation
CORE will include a suite of automated tests that can be used to help confirm that a CORE implementation is functioning correctly. CDISC also plans to implement a certification program to verify that software that executes the Conformance Rules functions correctly and consistent with the CORE Reference Implementation.
CDISC plans to implement a certification program to verify that any software that executes the Conformance Rules functions correctly and consistent with the CORE Reference Implementation. All CORE Certified software engines will demonstrate that they yield the same results given the same set of rules and CDISC datasets.
Data Security
CORE has undergone a security review and comprehensive threat assessment to identify and address vulnerabilities. The CORE architecture addresses stringent data and application security requirements to ensure that CORE can be deployed in a highly-secured and validated environment. Since CDISC will not provide a production deployment of CORE, each implementation should be evaluated to ensure the environment meets all identified security requirements. Given the detailed nature of software and data security, CDISC plans to publish a white paper to provide a comprehensive response to address CORE security questions.
Miscellaneous
Going forward, the development cycle for the Conformance Rules will become a normal part of the standards development.
- CORE will initially only be developed as a CLI and will ingest locally maintained data.
- The first software vendors have released a free version of desktop application.
- Depending on community request, CDISC can create a simple user interface but will highly depend on community engagement and support.
- The CORE engine will be designed to run conformance rules from the CDISC Library, as well as sponsor-defined rules. Sponsor-defined rules will not be maintained in the CDISC Library.
- CORE plans to include Conformance Rule authoring tools that support the development and testing of both CDISC and sponsor-defined rules.
Latest page content update: 17 Jan 2024
CORE Engine Reference Implementation in GitHub
The CORE Engine Reference Implementation is the current version of the Engine. The CORE Engine Reference Implementation has been transitioned to the open-source environment with its provision on GitHub. The GitHub-based Engine is:
- Provided as open source with the permissive MIT license
- Registered with the CDISC Open-Source Alliance (COSA)
- Available to users for free
- Provided with a command line interface (CLI)
- Accessed at the GitHub CDISC-rules-engine repository, including special instructions in the Readme file
The CORE Engine Reference Implementation on GitHub is an advancement over the CORE Minimum Viable Product (MVP) Engine early evaluation version that was previously available via the CORE web page. In particular, the GitHub-based Engine is updated to process additional Rule syntax (YAML) engaged to handle new processing conditions that were encountered as the CORE team developed additional Rules.
The GitHub-based Engine is provided with a command line interface (CLI) and not with a Graphic User Interface (UI). The earlier MVP version had been provided with a basic UI to allow users to quickly and easily run the Engine to provide early feedback on the Engine functionality to the CORE team. Provision with a CLI provides implementers with the maximum flexibility while integrating the CORE Engine into their processing environments.
The GitHub-based Engine with its CLI, in general, has functionality like that seen in the earlier MVP version. A pre-recorded CORE Tutorial, which was prepared while running the earlier MVP Engine version, demonstrates the basic Engine functionality that is available in the GitHub-based version. You can view that tutorial just below on this page.
View the CORE Tutorial
Copy here.