ADaM
Collaborators
Summary
Use Case

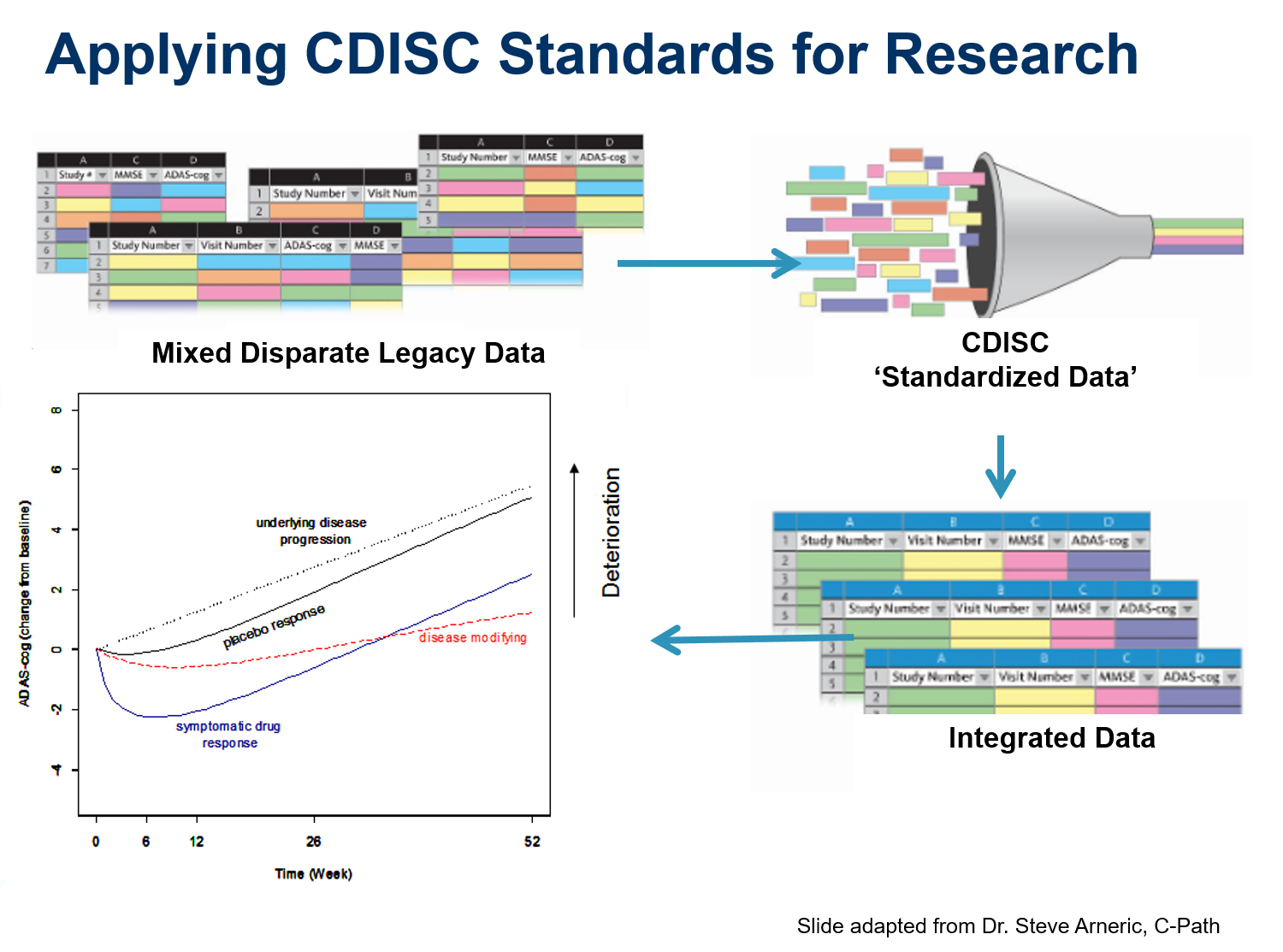
Cytel converted data from Unilever’s cosmetics trials to CDISC SDTM standards with the long-term goal of combining the data from all human trials into a single database using an industry standard format.
LAB
Collaborators
Summary
Use Case
One of the largest components of clinical trial data is laboratory data. Developing a standard lab data format is critical to achieving CDISC’s mission of creating standard data models that support the end-to-end data flow of clinical trials, from the data sources into an operational database and through to analysis and submission.
ODM
Collaborators
Summary
Use Case

The TRANSFoRm eHealth solution for clinical trial management collects Patient Reported Outcome Measurement (PROM) data from web and mobile (mHealth) platforms, CRF data extracted from electronic health records (EHRs), and data entered into CRFs by researchers. The PROM software permits remote trial data collection from patients suffering from chronic diseases, and uses algorithms to trigger alarms if the patients need medical attention. The ability to provide this service remotely reduces healthcare costs, while improving quality of life by minimizing disruption to patients’ lives. The system relies on the ODM standard to generate its questionnaire-based, data-collection instruments, including mHealth forms running on Apple’s iOS-based iPhones or Android phones.
PRM
Collaborators
Summary
Use Case
CDISC served as a key stakeholder, providing input to the development of Common Protocol Template (CPT). CPT aligns with NIH/FDA-developed Template connecting the parallel universes of clinical care and research as stated by the FDA Commissioner during the CDISC/FDA strategy session in August 2016.
SDTM
Collaborators
Summary
Use Case
The Immunology Database and Analysis Portal (ImmPort) has been developed under the Bioinformatics Integration Support Contract (BISC) Phase II by the Northrop Grumman Information Technology Health Solutions team for the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Division of Allergy, Immunology, and Transplantation (DAIT). Immport is based on SDTM and is the largest database of data flow.
Alzheimer's
Collaborators
Summary
Use Case





The Coalition Against Major Diseases (CAMD) used CDISC standards to develop a database that enables pooling of data from different sources to create an Online Data Repository for Alzheimer’s with the aim of supporting accelerated drug development.
Alzheimer’s Disease trial simulation tool was built using CDISC standardized data allows researchers to optimize the design of new trials.
Crohn's Disease
Collaborators
Summary
Use Case

CDISC is developing the first global, non-proprietary clinical data standards for Crohn’s disease with a grant award of $1 million from The Leona M. and Harry B. Helmsley Charitable Trust.
The Crohn’s disease award is part of Helmsley’s $5.6 million commitment to CDISC announced previously and will benefit the Crohn’s disease field by improving the quality, usefulness, consistency, sharing and mutual understanding of clinical data. Once developed, the clinical data standards will increase the efficiency and collaboration within research and clinical development to better address the unmet medical needs of patients. These standards will also provide the tools to further support Helmsley's data sharing priorities.
Malaria
Collaborators
Summary
Use Case


A team from the Worldwide Antimalarial Resistance Network (WWARN), University of Oxford, and Infectious Diseases Data Observatory (IDDO) led by Dr. Laura Merson applied CDISC standards in field research data collection and aggregation leading to new treatment recommendations on how low-weight children suffering from malaria in sub-Saharan Africa are treated. These updated guidelines included increasing dosing for this pediatric weight class resulting in a reduction of mortality in these vulnerable patients.
Multiple Sclerosis
Collaborators
Summary
Use Case


The Multiple Sclerosis Outcomes Assessments Consortium (the Critical Path Institute and the National MS Society) use CDISC standards to standardize and analyze MS data to qualify a new measure of disability as a primary or secondary endpoint for future trials of MS therapies
Parkinson's Disease
Collaborators
Summary
Use Case


Application of CDISC Standards has supported the discovery of clinical research biomarkers for Parkinson’s Disease — the evidence for which had been hidden in non-standardized datasets for years prior to their standardization, aggregation, and analysis. These biomarkers now enable researchers to identify diseases early, when intervention may be possible.
Polycystic Kidney Disease
Collaborators
Summary
Use Case

Application of CDISC Standards has supported the discovery of clinical research biomarkers for polycystic kidney disease —the evidence for which had been hidden in non-standardized datasets for years prior to their standardization, aggregation, and analysis. These biomarkers now enable researchers to identify diseases early, when intervention may be possible.
Post Traumatic Stress Disorder
Collaborators
Summary
Use Case

TAUG-PTSD v1.0 was funded by Cohen Veterans Bioscience as part of their vision of improving data sharing and open science tools for global research in the field of PTSD. The Cohen organization was one of the first adopters of the standard as they have based their BRAIN Commons Platform on CDISC standards.
Traumatic Brain Injury
Collaborators
Summary
Use Case

CDISC developed a standard for Traumatic Brain Injury under the Coalition for Accelerating Standards and Therapies (CFAST) initiative, a collaboration with C-Path, with expert contribution from members of the TBI Endpoints Development (TED) Initiative and Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) investigator communities, and generous support from One Mind for Research.
The CDISC TBI standards will help catalyze awareness of the types of clinical information that may be relevant to capture, in terms of clinical measures and biomarkers in TBI studies
Tuberculosis
Collaborators
Summary
Use Case

The Critical Path Institute used CDISC standards to create an aggregated TB Clinical Trial Data-Sharing Platform to enable more efficient and effective drug development
Pancreatic Cancer
Collaborators
Summary
Use Case
TAUG-PaCa was developed in partnership with, and funded by, the Pancreatic Cancer Action Network (PanCAN), the leading pancreatic cancer patient advocacy organization.
Diabetes Type 1 - Exercise and Nutrition
Collaborators
Summary
Use Case

CDISC has been awarded a grant in the amount of $1,000,000 by The Leona M. and Harry B. Helmsley Charitable Trust to develop Type 1 Diabetes (T1D)-related data standards. The Helmsley grant will make possible the development of new T1D CDISC standards that will extend published diabetes standards by developing machine-readable metadata in the following focus areas: Pediatrics, Devices, Prevention, and Exercise.
Diabetes Type 1 - Pediatrics and Devices
Collaborators
Summary
Use Case

CDISC has been awarded a grant in the amount of $1,000,000 by The Leona M. and Harry B. Helmsley Charitable Trust to develop Type 1 Diabetes (T1D)-related data standards. The Helmsley grant will make possible the development of new T1D CDISC standards that will extend published diabetes standards by developing machine-readable metadata in the following focus areas: Pediatrics, Devices, Prevention, and Exercise.
Diabetes Type 1 - Screening, Staging and Monitoring of Pre-clinical Type 1 Diabetes
Collaborators
Summary
Use Case

CDISC has been awarded a grant in the amount of $1,000,000 by The Leona M. and Harry B. Helmsley Charitable Trust to develop Type 1 Diabetes (T1D)-related data standards. The Helmsley grant will make possible the development of new T1D CDISC standards that will extend published diabetes standards by developing machine-readable metadata in the following focus areas: Pediatrics, Devices, Prevention, and Exercise.
Pediatrics
Collaborators
Summary
Use Case

This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777389. The Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. CDISC would like to recognize the support of the subject matter experts from the consortium in development of this User Guide.
Partnerships - Use Cases