
Therapeutic Area User Guide for Post Traumatic Stress Disorder
Version 1.0 (Final)
Notes to Readers
- This is version 1.0 of the Therapeutic Area User Guide for Post Traumatic Stress Disorder.
- This document is based on SDTM v1.7, SDTMIG v3.3, SDTMIG-MD v1.1, CDASHIG v2.0, and ADaM v2.1
Revision History
| Date | Version |
|---|---|
| 2018-12-12 | 1.0 Final |
© 2018 Clinical Data Interchange Standards Consortium, Inc. All rights reserved.
Contents
- Appendices
- Appendix A: PTSD Team
- Appendix B: Glossary and Abbreviations
- Appendix C: Non-Standard Variables
- Appendix D: References
- Appendix E: Representations and Warranties, Limitations of Liability, and Disclaimers
1 Introduction
This Therapeutic Area Data Standards User Guide for Post Traumatic Stress Disorder (TAUG-PTSD) v1.0 was developed under the Coalition for Accelerating Standards and Therapies (CFAST) initiative. This data standard follows several previously published neuroscience-related TAUGs, including Alzheimer's disease, schizophrenia, traumatic brain injury, and major depressive disorder. The TAUG-PTSD was funded by Cohen Veterans Bioscience, Inc as part of the collaborative efforts between Cohen Veterans Bioscience and the Clinical Data Interchange Standards Consortium (CDISC).
The purpose of this TAUG-PTSD is to describe how to use CDISC standards to represent data pertaining to post traumatic stress disorder studies in adult and pediatric populations. This first version (v1.0) focuses on the representation of data using the Study Data Tabulation Model (SDTM), and the Study Data Tabulation Model Implementation Guide (SDTMIG) as well as some Clinical Data Acquisition Standards Harmonization (CDASH) and Analysis Data Model (ADaM) representation.
1.1 How to Read this Document
- First, read the SDTM v1.7, SDTMIG v3.3, SDTMIG-MD v1.1, CDASHIG v2.0, and ADaM v2.1. These standards are available at http://www.cdisc.org/.
- Next, read Introduction to Therapeutic Area Standards (at http://wiki.cdisc.org/x/SSy8AQ) and/or take CDISC's free training module TA001 - Overview of Therapeutic Area User Guides (at CDISC Online Training) to be sure to know what to expect from a therapeutic area user guide.
- Read this guide all the way through (without skipping any sections) at least once.
- Finally, revisit any sections of particular interest.
All general caveats for TA standards given in the Introduction to Therapeutic Area Standards (http://wiki.cdisc.org/x/SSy8AQ) apply to this document.
Some things to bear in mind while reading this document:
- This document does not replace or supersede the foundational CDISC standards or their implementation guides, and should not be used as a substitute for any other CDISC standard.
- This document does not repeat content already published in another CDISC standard.
- This document is not and does not try to be an exhaustive documentation of every possible kind of data that could be collected in relation to post traumatic stress disorder.
- The advice and examples presented in this document are influenced by ongoing internal standards development at CDISC. If a modeling approach seems inconsistent with a published standard, it may be a genuine error, but it could also be a reflection of potential or upcoming changes to the standard.
- The examples in this document use CDISC Controlled Terminology (CT) where possible, but some values that seem to be controlled terminology may still be under development at the time of publication, or even especially plausible "best-guess" placeholder values. Do not rely on any source other than the CDISC value set in the NCI Thesaurus (available at http://www.cancer.gov/research/resources/terminology/cdisc) for controlled terminology.
1.2 Organization of this Document
This document is divided into the following sections:
- Section 1, Introduction, provides an overall introduction to the purpose and goals of the Post Traumatic Stress Disorder Therapeutic Area Standard User Guide.
- Section 2, Subject and Disease Characteristics, covers data that are usually collected once at the beginning of a study.
- Section 3, Disease Assessments, covers data that are typically collected multiple times over the course of the study.
- Section 4, Routine Data, covers data that are collected in most PTSD studies.
- Section 5, Analysis Data, includes key data analysis concepts for a PTSD study.
- Appendices provide additional background material and describe other supplemental material relevant to PTSD.
1.3 CDASH Metadata and Annotated CRFs
CDASH examples include both metadata tables and sample case report forms (CRFs). Each table of CDASH metadata corresponds to an example annotated CRF (aCRF), built directly from the metadata. The annotations show the variables associated with each field in the context of data collection (CDASH) and submission (SDTM) and are denoted by color. Data that are collected using the same variable name as defined in the SDTMIG are in RED. If the CDASHIG variable differs from the one defined in the SDTMIG, the CDASHIG variable is in GREY. Data collected but not submitted in SDTM-based datasets are denoted as NOT SUBMITTED The CDASH variable ----NO (e.g., --AENO, --PRNO) are generically annotated as ASSOCIATE WITH RELATED RECORD VIA RELREC. These RELREC relationships are sponsor-defined and depend on the actual study and the procedures used by the sponsor to create RELREC.
The following diagram illustrates how to interpret the annotations.
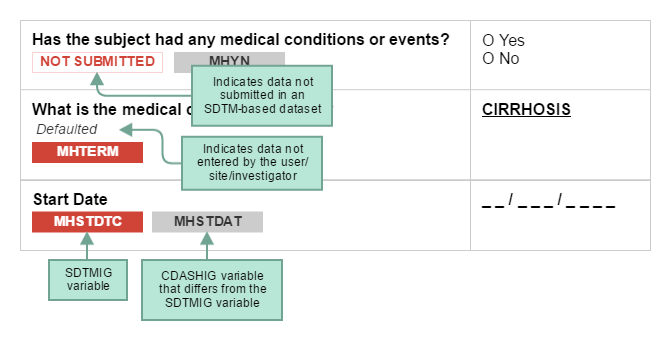
When viewing sample CRFs and aCRFs, bear in mind that:
-
When implementing CDASH in a denormalized structure, denormalized CDASH variable names are created by the sponsor, when needed. Denormalized CDASH variable names for TAUGs generally use the following naming convention:
< Topic Variable values >_<Qualifier(s)>_<SDTMIG Target >.Sponsors may define their own conventions for creating denormalized CDASH variable names. Examples:
- DIABP_VSORRES, where DIABP is the value for VSTESTCD (topic variable) and VSORRES is the SDTMIG target.
- DIABP_ARM _RIGHT_VSORRES, where DIABP is the value for VSTESTCD (topic variable); ARM and RIGHT are values of the SDTM Qualifier variables VSLOC and VSLAT:VSORRES is the SDTMIG target.
- Depression_MHOCCUR, where Depression is the value of MHTERM (topic variable); MHOCCUR is the SDTMIG target.
- More information may also be found in the CDASH Model and CDASHIG.
- Example CRFs are provided to illustrate data collection instruments. These are only examples and are not meant to recommend any particular layout over another.
- Example CRFs include instructions for the clinical site regarding how to enter collected information on the CRF.
- Example CRFs are best understood in conjunction with their respective metadata tables and/or the CDASH Domain Metadata Tables.
- Most example CRFs do not include the Highly Recommended header variables. The population of these values is usually determined by the sponsor's data management system.
- Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
- CDASH variable names for denormalized variables are examples. Sponsors may use other conventions for creating denormalized CDASH variable names.
- CDASH variable names that are annotated as "NOT SUBMITTED" may be used to contribute towards the population of other appropriate variables when the SDTM-based datasets are created.
- CDASH variables may also be mapped to or used to populate other SDTMIG variables that are not shown.
- Although a CDASH variable usually maps to a single SDTM-based domain, some CRFs may illustrate mapping to multiple variables as indicated by SDTM annotations such as TUDTC AND TRDTC. CDASH variables may also be mapped to or used to populate other SDTMIG variables that are not shown.
1.4 Known Issues
- --TESTCD/--TEST: When controlled terminology for tests is developed, there is often debate about how much information related to the test should be incorporated into the --TEST and --TESTCD variables, and how much should be represented in other variables. For example, the pre-coordination of test qualifiers such as "mean" is still under discussion. The incorporation of qualifier information into the test definition is referred to as pre-coordination, whereas the representation of qualifier information in a separate qualifier variable is referred to as post-coordination. At the time of publication of this TAUG, some --TEST/--TESTCD values are preliminary; final approved terminology may be different.
- Large Volume of Raw Data:
- This TAUG presents examples of the representation in SDTM-based domains of derived data that was calculated by the external vendor. Representing these derived measures within an SDTM-based domain (e.g., sleep testing, startle response) is under consideration by the Submission Data Standards (SDS) and Analysis Data Model (ADaM) Teams and is subject to change.
-
All collected data that supports review, reporting, and analysis should be submitted. There are cases where a large volume of raw data is generated by a device many times per second (e.g., ECG waveform data, data collected by polysomnography). In this case, summary data that are received as an output from a device may be represented in an SDTM dataset (e.g., average over a fixed period of time) instead of the full set of the raw data. Even though summary data are submitted, health authorities may request the submission of all of the raw data from the device.
- Representation of Device properties in DU: Device set-up properties that are fixed for all subject using the same device can be represented by leaving the USUBJID null. However, when multiple testing sites are using the same device with different device set-up parameters, the set-up parameters must be provided for each subject. This may create duplicate information when all subjects at that test site are evaluated using the same set-up parameters.
- CDASH MHSTDAT, MHENDAT Question Text/Prompt: The MHSTDAT (MHENDAT) question text "What was the medical condition or event start (end) date?" and prompt "Start/End Date" in the CDASHIG does not consider the need to ask a specific question about Diagnosis Date or Symptom Start (End) Date. The example aCRF in Section 2.1, PTSD History - Symptoms and Diagnosis, uses the prompts Date and Start Date. CDASH is considering updating these Question Text and Prompts to allow the sponsor to include the values recorded in MHEVDTYP.
2 Subject and Disease Characteristics
Post traumatic stress disorder (PTSD) is a clinically diagnosed psychiatric condition that can occur following the experience or the witnessing of life-threatening events, such as military combat, natural disasters, terrorist incidents, serious accidents, or physical or sexual assault in adulthood or childhood. It is characterized by experiencing distress or fear in non-threatening situations, as well as disturbing thoughts or feelings related to the event, which can negatively affect relationships and impair functioning. A person may develop PTSD after directly experiencing or witnessing a traumatic event, or by being confronted with an event happening to someone close to the individual. Not everyone who has been exposed to a traumatic event will develop PTSD. According to the National Center for PTSD, about 7 or 8 out of every 100 people will experience PTSD at some point in their lives.[1]
Symptoms of PTSD include re-experiencing symptoms (e.g., flashbacks, frightening thoughts), avoidance symptoms (e.g., staying away from people, places, or things that trigger the memory of the traumatic event), hyperarousal and reactivity symptoms (e.g., easily startled or angered), and cognition and mood symptoms (e.g., negative thoughts or loss of interest in normal activities).[2] These symptoms often can be very debilitating and interfere with work and relationships and can continue to be experienced even when there is no perceivable danger or threat.
In general, information about subject and disease characteristics includes events and activities that have affected the subject prior to or at the start of the study. For PTSD, this includes basic demographic data, details about the trauma that the subject may have experienced, and baseline assessments as to the severity of the subject's PTSD. Commonly co-occurring physical and mental health conditions are often also collected, as well substance use information. Basic demographic data collected in PTSD studies generally include date of birth or age, sex, race, and ethnicity. Other socioeconomic characteristics such as years of education, education level, annual income, employment status, and marital status are typically collected. More complex measures of social status, social support, and social circumstances may also be collected. These more complex measures are often collected using published instruments (within CDISC, these are referred to as Questionnaires, Ratings, and Scales [QRS]). Because there are many challenges associated with the collection of information on social status and social circumstances (many that depend on the particular research goal of the study), no specific SDTM examples of these measures are provided in this TAUG. Typically published instruments would be modeled in appropriate QRS domains. Other characteristics that are collected but not based on a published measure (e.g., annual income, education level) may be represented in the Subject Characteristics (SC) domain. See the SDTMIG for additional information on the Demographics (DM), Subject Characteristics (SC), Medical History (MH), Substance Use (SU) domains, and the QRS domains.
Various published instruments may also be administered to collect information used to characterize the subject at the start of the study. These questionnaires are typically represented in the SDTM QS domain. These same instruments may be repeated during the study (See Section 3.2, Questionnaires, Ratings, and Scales).
2.1 PTSD History - Symptoms and Diagnosis
The American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM–5) is the most widely accepted nomenclature used by clinicians and researchers for the classification of mental disorders. PTSD is included in a new category in DSM–5, Trauma- and Stressor-Related Disorders;[3] full copyrighted criteria are available from the American Psychiatric Association. The diagnosis of PTSD requires exposure to a traumatic or threatening event, either by directly experiencing or witnessing the event, and symptoms that last more than a month.
Example
This annotated case report form (aCRF) illustrates the collection of symptoms and diagnosis of PTSD in a study. This study used the DSM–5 criteria.[4] See Section 1.3, CDASH Metadata and Annotated CRFs, for explanation of annotations.
The Medical History (MH) domain is used to represent these symptoms and diagnosis of PTSD.
In this example illustrating symptoms and diagnosis of PTSD for a subject in a study using the DSM–5 criteria, the study collected symptoms as well as the PTSD diagnosis. MHGRPID was used to group the symptoms with the diagnosis. Medical History Event Date Type (MHEVDTYP) was used to indicate that the aspect of the condition by which MHSTDTC is defined is the diagnosis of PTSD.
3 Disease Assessments
Disease assessments are typically done repeatedly over the course of post traumatic stress disorder (PTSD) studies and are used to evaluate how the subject is progressing. The assessments most commonly used to determine the course of PTSD are various questionnaires, scales, and instruments. (See Section 3.2, Questionnaires, Ratings, and Scales, for a list of those identified for development as part of this TAUG.) These are often used to assess the severity of PTSD symptoms. Physiological testing (e.g., skin conductance, startle response) may also be performed. In their article, Hamilton et al provided a catalog of measures for use in PTSD research. [4]
3.1 Neuroimaging
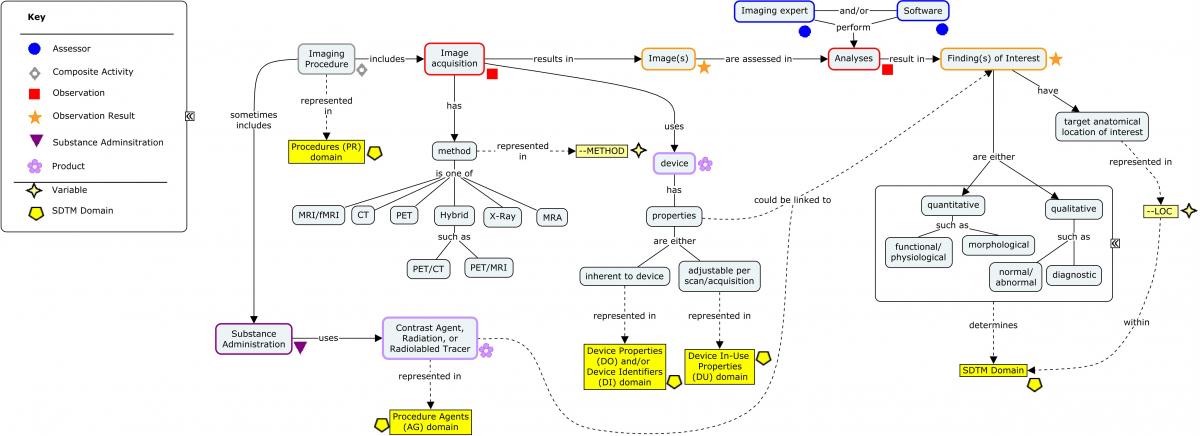
The brain circuitry of PTSD has been studied using various imaging modalities which can help to identify damage to the brain. Brain regions that are thought to be important include the hippocampus, amygdala, and medial prefrontal cortex. Imaging of the brain may be performed to assess the anatomic features of the brain and to assess the functioning of the brain during specific tasks (e.g., response to a stimuli). Magnetic resonance imaging (MRI) and computed tomography (CT) scans are often used to assess the structural features of the brain; positron emission tomography (PET), regional cerebral blood flow (rCBF), and functional magnetic resonance imaging (fMRI) are used to assess the functioning of the brain.
The following concept map shows the various components of an imaging evaluation and where this information is stored in SDTM. SDTM imaging examples can be found in several previously published TAUGs, including Huntington's Disease and Traumatic Brain Injury.
3.2 Questionnaires, Ratings, and Scales
Questionnaires, Ratings, and Scales (QRS) are maintained as stand-alone supplements on the CDISC website at http://cdisc.org/qrs. The following table lists assessments that are being pursued as potential supplements to the SDTMIG as part of the development work for Version 1.0 of the TAUG-PTSD. Supplements may or may not be finalized at the time of publication of this document, and depend on copyright approval where applicable. CDISC cannot produce supplements for copyrighted measures without the express permission of the copyright holder.
Sponsors should refer to the CDISC website (https://www.cdisc.org/foundational/qrs) if a measure of interest is not included below, as it may have been developed for another TAUG. New measures are implemented on an ongoing basis by the CDISC QRS Terminology and Standards Development sub-teams.
QRS Supplements of Interest to PTSD
| Full Name and Abbreviation | Copyright Permission Status | Supplement Status |
|---|---|---|
| Child PTSD Symptom Scale (CPSS) | To be requested | |
| Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) | Granted | Terminology in progress |
| Clinician-Administered PTSD Scale for DSM-5 – Child/Adolescent Version (CAPS CA-5) | To be requested | |
| Combat Exposure Scale (CES) | Public domain | Supplement in progress |
| Deployment Risk and Resilience Inventory-2 (DRRI-2) | Public domain | Supplement in progress |
| Dot-Probe Task | Public domain | Terminology in progress |
| Emory Treatment Resistance Interview for PTSD (E-TRIP) | Granted | Terminology in progress |
| Impact of Events Scale - Revised (IES-R) | To be requested | |
| Life Events Checklist for DSM-5 (LEC-5) | Public domain | Supplement in progress |
| Modified PTSD Symptom Scale (MPSS-SR) | To be requested | |
| Pittsburgh Sleep Quality Index (PSQI) | To be requested | |
| PTSD Checklist for DSM-5 (PCL-5) | Public domain | Supplement in progress |
| Social Support Survey Instrument | Public domain | Supplement in progress |
| Trauma Symptom Checklist for Children (TSCC) | Granted | Supplement in progress |
| Trauma Symptom Checklist for Young Children (TSCYC) | Granted | Supplement in progress |
| Traumatic Events Screening Inventory for Children (TESI-C) – Child Report | Public domain | Terminology in progress |
| Traumatic Events Screening Inventory for Children – Parent Report (TESI-PR-R) | Public domain | Terminology in progress |
| UCLA Child/Adolescent PTSD Reaction Index for DSM-5 – Child Version | To be requested | |
| UCLA Child/Adolescent PTSD Reaction Index for DSM-5 – Parent Version | To be requested | |
| Walter Reed Army Medical Center Blast Injury Questionnaire | To be requested |
3.3 Skin Conductance
Skin conductance (SC)—also known as galvanic skin response, electrodermal response, psychogalvanic reflex, skin conductance response, or skin conductance level—is a method of measuring the electrical conductance of the skin. Skin conductance is used as an indication of psychological or physiological arousal. When sweat gland activity increases, there is an increase in skin conductance. Skin conductance can be used as a measure of emotional and sympathetic nervous system responses with spontaneous fluctuations or reactions to stimuli. Skin conductance is often measured in persons with PTSD, who often exhibit stronger skin conductance when asked questions regarding a traumatic event or asked to imagine a traumatic event they have experienced. Skin conductance is normally measured with electrodes positioned on the index and middle fingers. The specific research protocol describes how the skin conductance test is administered and analyzed.
Baseline and trauma challenge psychophysiological recordings are used to measure skin conductance and heart rate in response to a Trauma Interview and Trauma Imagery in order to determine changes in these values (see concept map below). The protocol for a skin conductance test consists of a Baseline phase and a Trauma Challenge phase (includes Trauma Interview and Trauma Imagery). During the Baseline phase, skin conductance and heart rate are monitored continuously while the participant rests quietly. During the first part of the Trauma Challenge phase, heart rate and skin conductance are continuously measured while the participant is administered a 21-question, open-ended trauma interview. After the interview, skin conductance and heart rate are monitored continuously while the participant is asked to think about and imagine the traumatic event. The full protocol for the administration of this measurement is available at https://www.phenxtoolkit.org/protocols/view/630901.[3] Any skin conductance device that records continuously at a rate of 5 samples per second or higher and any h eart rate measurement device that records continuously at a rate of 20 samples per second or higher may be used.
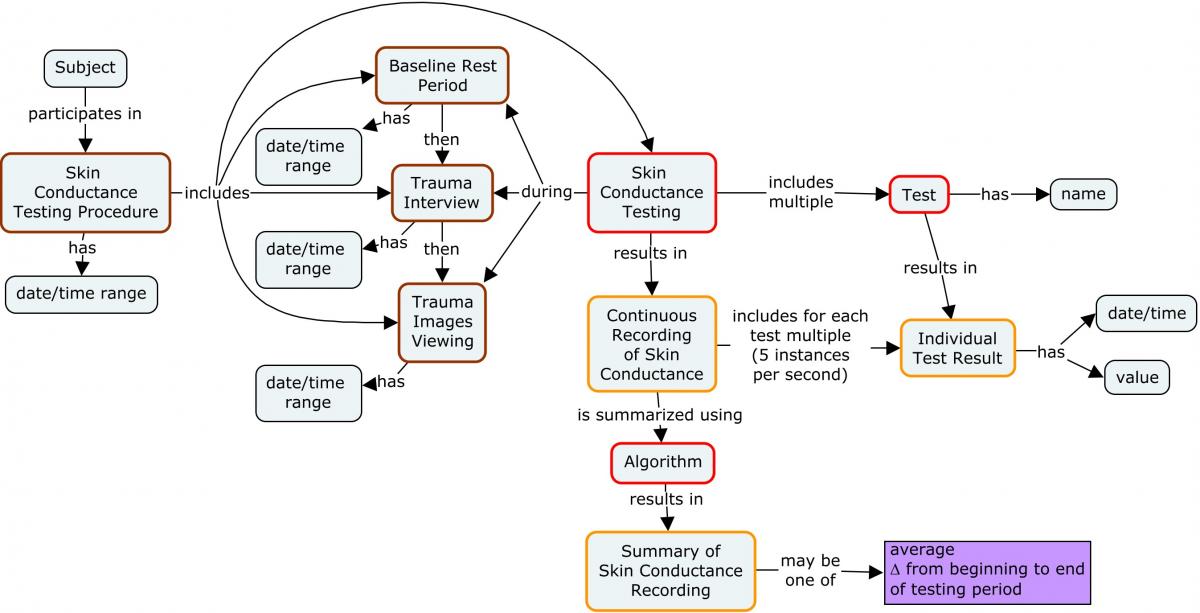
A sponsor should represent the collected skin conductance data in an appropriate SDTMIG domain and any derived data in an appropriate ADaM dataset. Example 1 shows how the actual collected data may be represented in an SDTMIG domain.
Example
In this study, the sponsor represented the actual Skin Conductance measurements in the Nervous System Findings (NV) domain. If the Heart Rate measurements were also collected, they would be represented in the Vital Signs (VS) domain. A key principle of CDISC standards is that the same data concepts are always represented using the same domain. The sponsor represented each of the Skin Conductance measurements during each phase of interest: Test Baseline, Trauma Interview, and Trauma Imagery. The measurements were obtained approximately 5 times per second. This assessment was performed at the screening visit and at the final visit in the clinical trial. The derived parameters used in the study analyses were computed by the sponsor and presented in an ADaM dataset. (See Section 5, Analysis Data, for an ADaM example.) The same device was used for all subjects in the clinical trial. The sponsor elected not to submit the Skin Conductance device information, as it was not of interest for this study. For an example of how device information may be represented, see Section 3.5, Sleep Studies, Example 1.
The sponsor collected the information on the actual start/end dates/times associated with each phase of the Baseline and Trauma Challenge Psychophysiological Recordings. This information was represented in the Procedures (PR) domain. The information is linked to the results in the NV domain using --LNKID. PRLNKID is populated with B1 for the first baseline time period, TINT1 for the first trauma interview period, TIMG1 for the first trauma imagery period, and B(n), TINT(n) and TIMG(n) for each subsequent testing phase at subsequent visits. In this example, the sponsor chose to use PRLNKID to link this dataset to the NV dataset.
The sponsor elected to include the name of the psychophysiological test in NVCAT. NVTPT was used to represent the different phases of the testing (e.g., Baseline, Trauma Interview, Trauma Imagery). NVTPTNUM was assigned 1, 2, or 3 to represent each of the phases. The sponsor represented the actual time of each Subject Characteristics (SC) measurement. Because these SC measurements were repeated 5 times per second, the sponsor used NVREPNUM to sequentially number these repeated measurements within each phase . Skin Conductance measurements are represented using uSiemens units. This information is linked to the results represented in the PR domain using --LNKID. NVLNKID is populated with B1 for the first Baseline phase, TINT1 for the first Trauma Interview phase, TIMG1 for the first Trauma Imagery phase, and B(n), TINT(n) and TIMG(n) for each subsequent testing phase at subsequent visits. In this example, the sponsor chose to use NVLNKID to link this dataset to the PR dataset.
Relationships between datasets are defined in RELREC. The following RELREC table shows how individual interventions in PR are related to the multiple skin conductance measurements in NV. IDVAR is used as the join key. Both USUBJID and IDVARVAL are null when representing dataset-to-dataset relationships. A unique PRLNKID was assigned to each phase of the Baseline and Trauma Challenge Psychophysiological Recordings at each visit and are linked with the same NVLNKID value to obtain all the SC results related to the phase.
3.4 Startle Response
PTSD patients have been reported to have a reduced capacity to suppress fear under safe conditions. A startle response test, which is a psychophysiological test, is used to investigate this reduced capacity to suppress fear. The startle response is measured by exposing the subject to various danger and safety cues and measuring recordings of the eyeblink muscle contraction using an electromyogram (EMG). The following concept map provides an overview of the measurement of the startle response. An individual study would typically have a protocol describing the stimuli, the number of times the set of stimuli are repeated (blocks) during the testing session, and the number of times within each block the specific stimuli are repeated (trials).
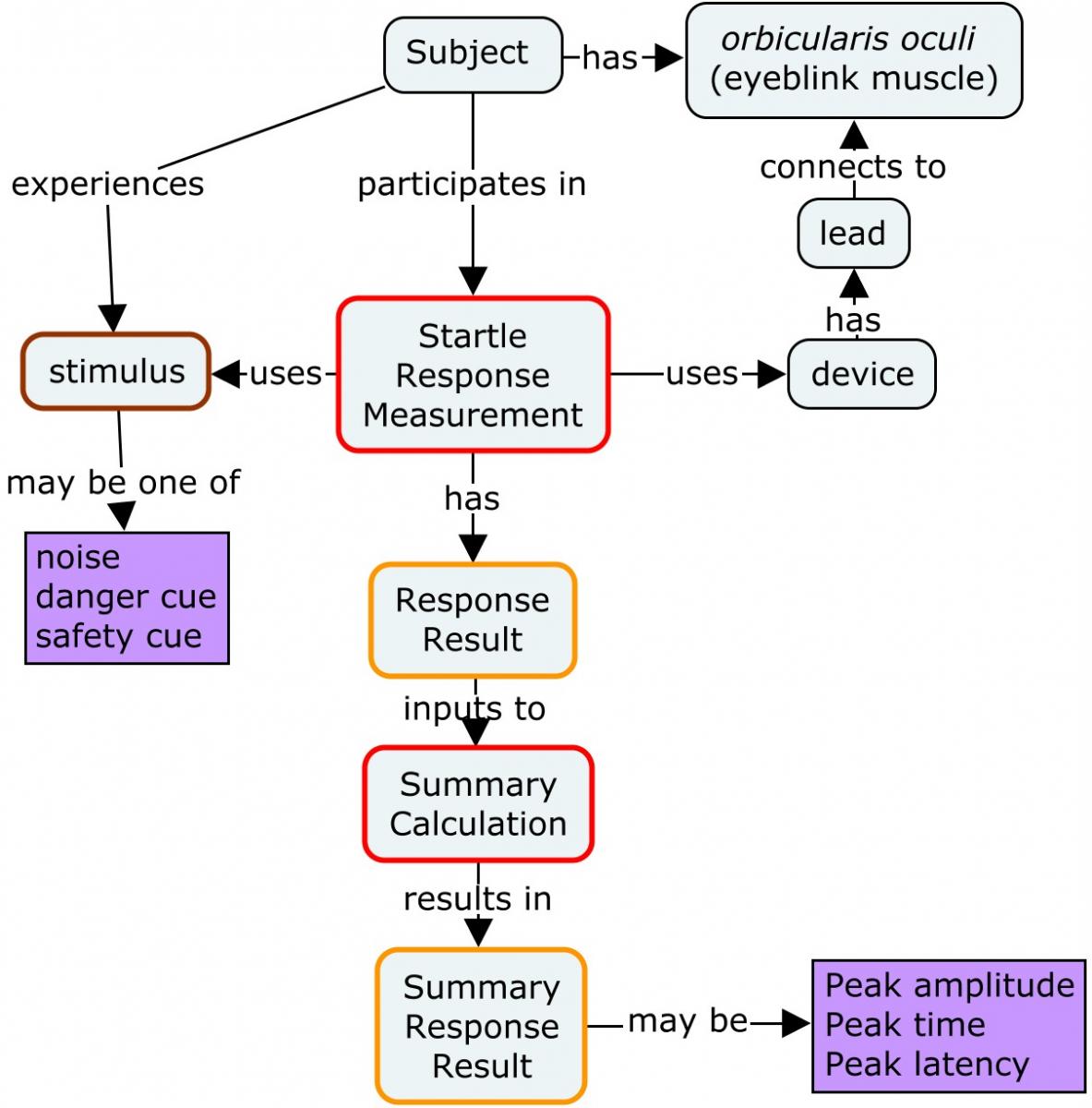
All collected data that supports review, reporting, and analysis should be submitted. There are cases where a large volume of raw data is generated by a device many times per second (e.g., data collected during startle response tests). In such cases, summary data that are received as an output from a device may be represented in an SDTM dataset (e.g., average over a fixed period of time) instead of the full set of the raw data. Even though summary data are submitted, health authorities may request the submission of all of the raw data from the device. In Example 1, only the peak value is included in the dataset; in Example 2, the mean peak values are included.
Example
In this study, the investigator performed a startle response test which included a series of blocks. Within each block, repeated trials of different types (e.g., danger cue, safety cue, noise alone) were repeated.
The test started with a set of 3 trials using the noise-alone cue, called a habituation block. This was followed by 3 blocks of 12 trials each. Within each of these blocks, the danger cue, safety cue, and the noise-alone cue were repeated 4 times in random order (12 trials).
The investigator elected to represent the Peak Response Amplitude and the Peak Response Amplitude Latency for each trial. The data below show only the information for the Habituation Block and Blocks 1 and 3 for selected trials. (Note: Block 2 is not shown in this example, due to space limitations.) For the Habituation Block, 2 trials are shown. For the other blocks, 1 trial for each cue is shown; that is, 1 row for each cue type (danger, safety, noise alone) for each block for the Peak Response Amplitude (3 trials) and the Peak Response Amplitude Latency (3 trials). The non-standard variable NVBLOCK is used to identify what experimental unit is represented (e.g., the block name) and the standard variable NVREPNUM is used to identify the trial number within the block. The non-standard variable NVCUETYP is used to identify the type of cue used during the particular time period of the test. The sponsor also collected information on which eye (right, left) was used for testing. If collected, device information can be represented using the domains in the SDTMIG-MD v1.1. Note also that, although this example uses the non-standard qualifier NVBLOCK, a sponsor could use a standard variable (e.g, NVGRPID) for this purpose. This example does not use NVGRPID in order to avoid creating the impression that a --GRPID variable, which is a sponsor-defined variable, should be used for the particular purpose illustrated in this example.
Example
In this study, the investigator tested the subject's startle response to 3 different cues: noise alone, a danger cue, and a safety cue. The test started with a set of 3 trials using the noise-alone cue, called a habituation block. This was followed by 3 blocks of 12 trials each. Within each block, the danger cue, safety cue, and the noise alone cue were repeated 4 times in random order (12 trials). The sponsor elected to represent the derived data produced by standard software that was designed to post-process the data from the device. The device used was collected, but the related device domains (DI, DO) are not shown. The representation of this data in an SDTMIG domain and the --TESTCD/--TEST controlled terminology is under discussion (see Section 1.5, Known Issues). The derived data provided was the mean of the Peak Response Amplitude and mean of the Peak Response Amplitude Latency of the trials in each block for each type of cue. The NSV NVBLOCK is used to identify records within each block of the experiment. The NSV NVCUETYP is used to identify the type of cue used during this particular time period of the test. The NSV NVCOLSRT is used to indicate that the mean of the trials in each block for each cue are reported. NVDTC denotes the date of the test. Ellipsis are used to indicate rows of data that are not displayed for the different cues.
Note: Although this example uses the non-standard qualifier NVBLOCK, a sponsor could use a standard variable (e.g, NVGRPID) for this purpose. This example did not use NVGRPID in order to avoid creating the impression that a --GRPID variable, which is a sponsor-defined variable, should be used for the particular purpose illustrated in this example.
3.5 Sleep Studies
Subjects diagnosed with PTSD often report sleep problems. A polysomnography (PSG) test records certain body functions as the subject sleeps, and can be used to diagnose sleep disorders. A full PSG is typically conducted in a sleep center, but it may sometimes be conducted using a portable device. Tests are usually performed during a subject's normal sleep pattern. The PSG is used to diagnosis various sleep disorders (e.g., narcolepsy, obstructive sleep apnea, periodic limb movement disorders). A home sleep apnea test (HSAT) may also be used to evaluate sleep disorders. The PSG monitors many body functions, including brain activity, eye movements, muscle activity, heart rhythm, and respiratory functions. For information on standards for the use of these monitoring devices, see the Further Reading Sources in Appendix D, References.
The American Academy of Sleep Medicine's (AASM) Manual for the Scoring of Sleep and Associated Events (available at https://aasm.org/clinical-resources/scoring-manual/) is updated annually; when presenting sleep test results, it is important to document which version was used. The AASM manual provides rules for scoring sleep stages, arousal, respiratory events during sleep, movements during sleep, and cardiac events.
All collected data that supports review, reporting, and analysis should be submitted. There are cases where a large volume of raw data is generated by a device many times per second (e.g., data collected during PSG tests). In this case, summary data that are received as an output from a device may be represented in an SDTM dataset (e.g., average over a fixed period of time) instead of the full set of the raw data. Even though summary data are submitted, health authorities may request the submission of all of the raw data from the device.
Example
This is an example of a study where data is generated from a polysomnography (PSG) device to assess sleep disorders. Sleep studies provide information on how well subjects sleep and how a subject's body responds to difficulty with sleep. In this study, subjects were evaluated over 2 consecutive nights at an initial study visit, and then reassessed over 2 consecutive nights at the end of the study. Subjects at each investigator site selected which of a pre-selected set of sleep test centers to use. Hence, subjects at each investigator site may have attended different sleep centers. Subjects were required to use the same sleep testing center during the study. The sleep centers followed a similar protocol for conducting the sleep tests. However, because of the concerns of some subjects, the sleep centers modified the procedures as needed for each subject.
The example is split into 2 parts. The first part provides detailed information on how one could represent the relevant information about the PSG device being used for this testing. The second part of the example provides details on how to represent the clinical data generated from this PSG for the subject.
Two different PSG device models were used in the study, and the sponsor considered it important to specify information on the specific PSG model used during the study to allow data to be pooled with other studies. The representation of information on devices used to obtain study measurements or results is described in the SDTM Medical Device Implementation Guide (SDTMIG-MD), available at http://www.cdisc.org/.
The Device Identifiers (DI) domain is used to uniquely identify each specific device used in the study. This domain provides a consistent sponsor-defined variable (SPDEVID) for linking data across domains. At a minimum, Device Type (DIPARMCD="DEVTYPE") should be recorded. R efer to the SDTMIG-MD for guidance on terminology that should be used for DIVAL values when DIPARMCD is "DEVTYPE". For both PSG models, the specific device was classified as a Polysomnography analyzer (a device that includes electrodes, measuring instruments, samplers, filters, amplifiers, and appropriate software for processing data and providing patient diagnoses). The sponsor also provided information on Model and Manufacturer of the each device to allow each PSG device to be uniquely identified. Because both devices were manufactured by the same manufacturer, the model number is also included in DI. Note that this domain does not include USUBJID, as this represents information on the device itself and is not related to the subject.
The 2 PSG devices used in the study for obtaining measurements are assigned sponsor-defined SPDEVIDs. To uniquely identify the device, rows for DIPARMCD and DIPARM of Device Type, Model, and Manufacturer are provided.
The Device Properties (DO) domain is used to represent important characteristics of a PSG device that the sponsor wishes to represent but which do not form part of the unique sponsor-defined identification of the device provided in DI. These characteristics are properties of the device and can not be changed for each subject. In the example below, properties of the device that cannot change for each subject are represented in this domain (e.g., sensor type, sampling rate). Information on other channels, sample rates, and sensor type may also be included, but are not shown in this example. Sponsors may also include properties of a device that can be modified, if these properties are fixed by the sponsor for a particular study and cannot be modified for any subject.
The Device In-Use (DU) domain is used to represent the information on the set-up parameters of the PSG device used for each subject. This domain includes both USUBJID and SPDEVID. This domain is not intended to capture manufacturer setting (i.e., nominal) parameters, but rather the customized device settings for a given usage. Each parameter (i.e., setting) is represented by a separate row and is defined in the topic variable DUTESTCD. The value of the setting is represented in DUORRES. The settings are typically represented for each subject individually. In this example, the device settings were not the same across all subjects, hence the device setting must be provided for each subject. This approach may repeat the same device set-up parameters many times. This will be investigated further (see Section 1.5, Known Issues). The SDTMIG variables VISITNUM and DUDTC were included in the dataset as they are Expected, but they are left blank when the settings are not related to a specific subject testing on a specific date. If the device characteristics are changed at each visit, then the actual visit number would be used. For the DU example, it is assumed that the set-up for the PSG was the same for each subject at all assessments, including Night 1 and Night 2. If the PSG setup was changed between Night 1 and Night 2, the variables DUTPT and DUTPTNUM may be added to represent the PSG set-up for each night. The DU properties are shown below for 1 subject (40133) and select rows are shown for Subject 40136.
The Device-Subject Relationships (DR) domain links each subject to the associated devices. Information on only 2 subjects is provided as an example. This domain should be included when devices of interest are under study. When the devices are not under study, this domain may not be needed. If it is important to know the individual device used with each subject, it should be included. In this case, the sponsor included the DR domain.
Clinical Data The Procedures (PR) domain was used to represent the information on when and where the procedure was performed for each subject. However, in the Findings Observation Class, the test method may be represented in the --METHOD variable (e.g., electrophoresis, polymerase chain reaction). It is sometimes difficult to decide whether a diagnostic or therapeutic procedure (e.g., ultrasound, MRI, x-ray) should be represented as a separate record in the PR domain, or whether it should simply be represented as a test method qualifier (--METHOD) on the test result records represented in a Findings Observation Class domain. The following is recommended: If timing (e.g., start, end, duration) or an indicator populating PROCCUR, PRSTAT, or PRREASND is collected, then a PR record should be created. If only the findings from a procedure are collected, then --METHOD in the Findings domain(s) may be sufficient to represent the procedure; a related PR record is optional. It is at the sponsor's discretion whether to represent the procedure as both a test method (--METHOD) and related PR record.
The sponsor elected to represent the derived data produced by standard software that was designed to post-process the data from the device. The representation of this data in SDTM-based domains is under discussion (see Section 1.5, Known Issues ) . The test measurements were repeated for each night for each visit. Only the data for Night 1 for the screening session for Subject 40133 is provided below. Other data would be represented similarly. This example shows some of the main tests that would be assessed during a sleep test. These parameters include amounts and percentages of time spent in various sleep stages. NVTPT and NVTPTNUM are used to represent each night of the session. NVMETHOD is used to indicate that the test was performed using a polysomnography, and NVANMETH is used to indicate what scoring method was used for the sleep test. SPDEVID and NVLNKID are used to link these results to the appropriate dataset. SPDEVID shows which polysomnography analyzer was used (e.g., SPDEVID="PSG001"). In this example, the sponsor chose to use NVLNKID to link this dataset to the PR dataset. Other sponsors may choose to use other variables to link datasets. NVLNKID is assigned the value of A(n)N(n) to link this dataset to the PR dataset (e.g., A1N1=Assessment 1 Night 1). Other sponsors may choose to use other variables to link datasets. NVDTC and NVENDTC represent the timing of the testing (e.g., good night time, good morning time).
This is an example of the dataset relationships between the SDTM domains.
4 Routine Data
Some routinely collected data are not specific to a therapeutic area, such as data collected to assess the subject's history, substance use by the subject, and prior concomitant medications. The CDASH and SDTM foundational standards provide advice for the representation of such data. This section discusses how to represent data collected using the Emory Treatment Resistance Interview for PTSD (E-TRIP; available at https://pid.emory.edu/ark:/25593/p2wrj).[5]
Emory Treatment Resistance Interview for PTSD (E-TRIP)
E-TRIP was developed to evaluate previous treatment outcomes in PTSD. Treatments for PTSD may include psychotherapy or medications. Typical psychotherapy treatments include Prolonged Exposure (PE), Cognitive Processing Therapy (CPT), and Eye Movement Desensitization and Reprocessing (EMDR). Medication treatments may include approved selective-serotonin reuptake inhibitors (SSRIs), as well as off-label use of SSRIs, monoamine oxidase inhibitors (MAOIs), sedatives, and other antidepressants. C linician-administered questions are included to assess the adequacy and benefit derived from past treatment trials.[5]
E-TRIP is similar to other clinical classifications measures that have been described in the literature in that it uses a score that serves as a surrogate for, or ranking of, the disease status. The interviewer reviews and interprets a subject's status with respect the use of previous treatments for PTSD. The evaluations of each of the treatments by the interviewer is considered a clinical classification (represented in the Disease Response and Clin Classification (RS) domain), whereas the actual administration information for each treatment is represented in the Concomitant/Prior Medications (CM) and the Procedures (PR) domain.
Within CDISC, Clinical Classification instruments represented in the RS domain fall under the concept of Questionnaires, Ratings and Scales (QRS), and CDISC publishes standard QRS Supplements to the SDTMIG along with controlled terminology. Once generated, the Clinical Classifications Supplement is posted on the CDISC website (http://www.cdisc.org/qrs). Sponsors should always consult current QRS Supplements for guidance on submitting such data.
5 Analysis Data
This section illustrates the use of the Analysis Data Model (ADaM) to create a dataset to support the analysis of skin conductance. The example uses the measures collected as part of the Baseline and Trauma Challenge Psychophysiological Recordings described in Section 3.3, Skin Conductance. As previously noted, a high volume of collected values are generated when measuring skin conductance; using these individual measures directly for analyses is not practical. Instead, measures of skin conductance are often divided into pre-defined segments or time spans and then a summary measure (e.g., average, median, maximum) is determined for this segment. The summary measures for each segment are then used to derive analysis endpoints (e.g., changes in skin conductance over time). Different types of statistical methodologies and endpoints are applicable when analyzing skin conductance. The derived measures presented in this example are part of the protocol described by this vendor (available at https://www.phenxtoolkit.org/).[4]
As described in Section 3.3, Skin Conductance, skin conductance is measured during three defined phases: Baseline, Trauma Interview, and Trauma Imagery. According to the vendor, changes in skin conductance are of interest both within a phase and across phases. Therefore, baseline measures for each phase of the procedure need to be derived so that change from baseline can be calculated both within a phase and across phases. Example 1 uses the ADaM Basic Data Structure (BDS) to model these derived parameters using the collected skin conductance measures in the Nervous System Findings (NV) domain. Values of baseline are required to be defined within each phase of the interview, which requires the use of the BASETYPE variable to distinguish the set of records that utilize a given derivation of baseline.
This example is not intended to illustrate every possible variable that might be included in datasets created for statistical analysis of skin conductance. In addition to the selected variables shown in the example, a typical analysis dataset would contain additional subject-level variables, as well as variables to support traceability. In addition, this example is intended to be descriptive and illustrative of the use of the ADaM model, and should not be interpreted as the complete or the sole analysis requirements. The metadata and derivations presented are for illustrative purposes and attempt to closely follow the derivations described by the vendor. Refer to ADaM v2.1 and Version 1.1 of the ADaM Implementation Guide (ADaMIG) for required background about ADaM and the ADaM data structures.
Example 1
ADSCD Dataset Metadata
| Dataset | Description | Class | Structure | Purpose | Keys | Location | Documentation |
|---|---|---|---|---|---|---|---|
| ADSCD | Analysis of Skin Conductance | BASIC DATA STRUCTURE | One record per subject per basetype per parameter per analysis visit per analysis time point | Analysis | STUDYID, USUBJID, BASETYPE, PARAMCD, AVISIT, ATPT | ADSCD.xpt | ADSCD.SAS/SAP |
ADSCD Variable Metadata
| Variable | Label | Type | Controlled Terms or Codelist | Source / Derivation Comment |
|---|---|---|---|---|
| STUDYID | Study Identifier | text | Predecessor: ADSL.STUDYID | |
| USUBJID | Unique Subject Identifier | text | Predecessor: ADSL.USUBJID | |
| PARAM | Parameter | text | Assigned: Set to PARAMCD decode value | |
| PARAMCD | Parameter Code | text | Assigned: Based on parameter content | |
| AVISIT | Analysis Visit | text | BASELINE; MONTH 1 | Derived: Descriptive value for the timing of the visit |
| ATPT | Analysis Timepoint | text | FIRST MINUTE; LAST MINUTE; BASELINE | Derived: The value of analysis timepoint indicates the interval of time that was used to derive the average value of skin conductance. For this instrument, an interval of one minute is used for all BASETYPE besides for OVERALL. |
| AVAL | Analysis Value | float | Derived: See Parameter Value Level Metadata | |
| ABLFL | Baseline Record Flag | text | Derived: ABLFL=Y on the record that corresponds to the baseline value that is derived for the given PARAMCD and BASETYPE | |
| BASE | Baseline Value | float | Derived: AVAL where ABLFL=Y populated for all records of that PARAMCD and BASETYPE | |
| CHG | Change from Baseline | float | Derived: AVAL-BASE | |
| BASETYPE | Baseline Type | text | BASELINE PHASE FIRST MINUTE; TRAUMA INTERVIEW PHASE FIRST MINUTE; TRAUMA IMAGERY PHASE FIRST MINUTE; BASELINE PHASE OVERALL | Assigned: A description of the definition of baseline. For this instrument, the values of BASETYPE correspond to 4 definitions of baseline:
|
| ASTDTM | Analysis Start Datetime | datetime | Derived: ASTDTM is the numeric equivalent of NV.NVDTC that corresponds to the first record that is collected during the span of time described by ATPT. | |
| AENDTM | Analysis End Datetime | datetime | Derived: AENDTM is the numeric equivalent of NV.NVDTC that corresponds to the last record that is collected during to the span of time described by ATPT. |
PARAMCD [CL.PARAM]
| Permitted Value Code | Display Value (Decode) |
|---|---|
| AVGSCB | Average Skin Conductance Baseline (uSiemens) |
| AVGSCINT | Average Skin Conductance Trauma Interview (uSiemens) |
| AVGSCIMG | Average Skin Conductance Trauma Imagery (uSiemens) |
| DURINT | Duration of Trauma Interview (minutes) |
| AJCHGINT | Duration Adjusted Change from First to Last Minute Trauma Interview |
Parameter Value Level List - ADSCD [AVAL]
| Variable | Where | Type | Controlled Terms or Codelist | Source / Derivation Comment |
|---|---|---|---|---|
| AVAL | PARAMCD='AVGSCB' and ATPT='FIRST MINUTE' and BASETYPE='BASELINE PHASE FIRST MINUTE' | float | AVAL is the average of all values of NVSTRESN that were collected during the first minute of the Baseline Interview. The measurements falling within the first minute are determined by using first value of numeric(NVDTC) (where NVTPT='BASELINE') and accumulating all values of NVSTRESN that occur up to those with a datetime of numeric(NVDTC) + 60 seconds | |
| AVAL | PARAMCD='AVGSCB' and ATPT='LAST MINUTE' and BASETYPE='BASELINE PHASE FIRST MINUTE' | float | AVAL is the average of all values of NVSTRESN that were collected during the last minute of the Baseline Interview. The measurements falling within the last minute are determined by using the last value of numeric(NVDTC) (where NVTPT='BASELINE') and accumulating all values of NVSTRESN that occur after those with a datetime of numeric(NVDTC) - 60 seconds | |
| AVAL | PARAMCD='AVGSCINT' and ATPT='FIRST MINUTE' and BASETYPE='TRAUMA INTERVIEW PHASE FIRST MINUTE' | float | AVAL is the average of all values of NVSTRESN that were collected during the first minute of the Trauma Interview. The measurements falling within the first minute are determined by using first value of numeric(NVDTC) (where NVTPT='TRAUMA INTERVIEW') and accumulating all values of NVSTRESN that occur up to those with a datetime of numeric(NVDTC) + 60 seconds | |
| AVAL | PARAMCD='AVGSCINT' and ATPT='LAST' MINUTE' and BASETYPE='TRAUMA INTERVIEW PHASE FIRST MINUTE' | float | AVAL is the average of all values of NVSTRESN that were collected during the last minute of the Trauma Interview. The measurements falling within the last minute are determined by using the last value of numeric(NVDTC) (where NVTPT='TRAUMA INTERVIEW') and accumulating all values of NVSTRESN that occur after those with a datetime of numeric(NVDTC) - 60 seconds | |
| AVAL | PARAMCD='AVGSCIMG' and ATPT='FIRST MINUTE' and BASETYPE='TRAUMA IMAGERY PHASE FIRST MINUTE' | float | AVAL is the average of all values of NVSTRESN that were collected during the first minute of the Trauma Imagery. The measurements falling within the first minute are determined by using the first value of numeric(NVDTC) (where NVTPT='TRAUMA IMAGERY') and accumulating all values of NVSTRESN that occur up to those with a datetime of numeric(NVDTC) + 60 seconds | |
| AVAL | PARAMCD='AVGSCIMG' and ATPT='LAST' MINUTE' and BASETYPE='TRAUMA IMAGERY PHASE FIRST MINUTE' | float | AVAL is the average of all values of NVSTRESN that were collected during the last minute of the Trauma Imagery. The measurements falling within the last minute are determined by using the last value of numeric(NVDTC) (where NVTPT='TRAUMA IMAGERY') and accumulating all values of NVSTRESN that occur after those with a datetime of numeric(NVDTC) - 60 seconds | |
| AVAL | PARAMCD='AVGSCB' and ATPT=' ' and 'BASETYPE='BASELINE PHASE OVERALL' | float | AVAL is the average of all values of NVSTRESN where NVTPT='BASELINE' within AVISIT | |
| AVAL | PARAMCD= ' AVGSCINT' and ATPT='BASELINE' and BASETYPE='BASELINE PHASE OVERALL' | float | AVAL is set to AVAL where PARAMCD= ' AVGSCB' and ATPT=' ' and ' BASETYPE='BASELINE PHASE OVERALL' within AVISIT | |
| AVAL | PARAMCD='AVGSCINT' and ATPT=' ' and BASETYPE='BASELINE PHASE OVERALL' | float | AVAL is the average of all values of NVSTRESN where NVTPT='TRAUMA INTERVIEW' within AVISIT | |
| AVAL | PARAMCD= ' AVGSCIMG' and ATPT='BASELINE' and BASETYPE= ' BASELINE PHASE OVERALL' | float | AVAL is set to AVAL where PARAMCD= ' AVGSCB' and ATPT=' ' and ' BASETYPE='BASELINE PHASE OVERALL' within AVISIT | |
| AVAL | PARAMCD='AVGSCIMG' and ATPT=' ' and BASETYPE='BASELINE PHASE OVERALL' | float | AVAL is the average of all values of NVSTRESN where NVTPT='TRAUMA IMAGERY' within AVISIT | |
| AVAL | PARAMCD='DURINT' | float | AVAL is the difference between the first value of numeric(NVDTC) where NVTPT='TRAUMA INTERVIEW' and the last value of numeric(NVDTC) where NVTPT='TRAUMA INTERVIEW' divided by 60 to obtain minutes within AVISIT | |
| AVAL | PARAMCD='AJCHGINT' | float | AVAL is calculated as the value of CHG where PARAMCD='AVGSCINT' and ATPT='LAST MINUTE' and BASETYPE='TRAUMA INTERVIEW PHASE FIRST MINUTE' divided by AVAL where PARAMCD='DURINT' |
Appendices
Appendix A: PTSD Team
| Name | Institution/Organization |
|---|---|
| Amy Palmer, Team Lead | CDISC |
| Dana Booth | CDISC |
| Allyson Gage | Cohen Veterans Bioscience |
| Anne Germaine | University of Pittsburgh Medical Center |
| Tanja Jovanovic | Emory University |
| Patricia Kabitzke | Cohen Veterans Bioscience |
| Susan Kenny | Maximum Likelihood, Inc. |
| Kathleen Mellars | CDISC |
| Erin Muhlbradt | National Cancer Institute Enterprise Vocabulary Services |
| Carolyn Sartor | Yale University |
| Arieh Shalev | NYU Langone Medical Center |
| Olga Vovk | FITBIR |
| Jessica Wolfe | Cohen Veterans Bioscience |
Appendix B: Glossary and Abbreviations
| AASM | American Academy of Sleep Medicine |
| aCRF | Annotated Case Report Form |
| ADaM | Analysis Data Model |
| ADaMIG | ADaM Implementation Guide |
| CDASH | Clinical Data Acquisition Standards Harmonization Project |
| CDISC | Clinical Data Interchange Standards Consortium |
| CFAST | Coalition for Accelerating Standards and Therapies |
| Collected | Collected refers to information that is recorded and/or transmitted to the sponsor. This includes data entered by the site on CRFs/eCRFs as well as vendor data such as core lab data. This term is a synonym for captured. |
| Controlled Terminology (CT) | A finite set of values that represent the only allowed values for a data item. These values may be codes, text, or numeric. A code list is one type of controlled terminology. |
| CPT | Cognitive Processing Therapy |
| CRF/eCRF | Case report form (sometimes called a case record form or electronic case record form). A printed, optical, or electronic document designed to record all required information to be reported to the sponsor for each trial subject. |
| CT | Computed Tomography |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| Domain | A collection of observations with a topic-specific commonality about a subject |
| ECG | Electrocardiography |
| EEG | Electroencophalography |
| EMDR | Eye Movement Desensitization and Reprocessing |
| EMG | Electromyography |
| EOG | Electrooculography |
| E-TRIP | Emory Treatment Resistance Interview for PTSD |
| Foundational Standards | Used to refer to the suite of CDISC standards that describe the clinical study protocol (Protocol), design (Study Design), data collection (CDASH), laboratory work (Lab), analysis (ADaM), and data tabulation (SDTM and SEND). See http://www.cdisc.org/ for more information on each of these clinical data standards. |
| fMRI | Functional Magnetic Resonance Imaging |
| HSAT | Home Sleep Apnea Test |
| MAOI | Monoamine Oxidase Inhibitor |
| MRI | Magnetic Resonance Imaging |
| NCI | National Cancer Institute |
| NSV | Non-Standard Variable |
| Patient | A recipient of medical treatment |
| PE | Prolonged Exposure |
| PET | Positron Emission Tomography |
| PSG | Polysomnography |
| PTSD | Post Traumatic Stress Disorder |
| QRS | Questionnaires, Ratings, and Scales |
| rCBF | Regional Cerebral Blood Flow |
| SC | Skin Conductance |
| SDS | Submission Data Standards. Also the name of the team that maintains the SDTM and SDTMIG. |
| SDTM | Study Data Tabulation Model |
| SDTMIG | SDTM Implementation Guide (for Human Clinical Trials) |
| SEND | Standard for Exchange of Nonclinical Data |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| Subject | A participant in a study |
| TAUG | Therapeutic Area Data Standards User Guide |
Appendix C: Non-Standard Variables
The following table lists the non-standard variables used in this document, and gives their parent domain and variable-level metadata.
(Parenthesis indicates CDISC/NCI codelist)
Appendix D: References
Works Cited
- US Department of Veterans Affairs, PTSD: National Center for PTSD. How common is PTSD? https://www.ptsd.va.gov/public/PTSD-overview/basics/how-common-is-ptsd.asp. Updated October 3, 2016. Accessed September 17, 2018.
- National Institute of Mental Health. Post-traumatic stress disorder. https://www.nimh.nih.gov/health/topics/post-traumatic-stress-disorder-ptsd/index.shtml. Updated February 2016. Accessed September 17, 2018.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Hamilton CM, Strader LC, Pratt JG, et al. (2011) The PhenX toolkit: get the most from your measures. Am J Epidemiol. 2011 Aug 1;174(3):253-260. doi:10.1093/aje/kwr193.
- Dunlop BW, Kaye JL, Youngner C, Rothbaum B. Assessing treatment-resistant posttraumatic stress disorder: the Emory Treatment Resistance Interview for PTSD (E-TRIP). Behav Sci. 2014;4(4):511-527. https://pid.emory.edu/ark:/25593/p2wrj
Further Reading
-
Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406-422. doi:10.1093/sleep/20.6.406
-
Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association Report. Sleep. 1994;17:372-377.
- Rechtschaffen A, Kales A, eds. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects . Washington DC: Public Health Service, U.S. Government Printing Service; 1968.
Appendix E: Representations and Warranties, Limitations of Liability, and Disclaimers
CDISC Patent Disclaimers
It is possible that implementation of and compliance with this standard may require use of subject matter covered by patent rights. By publication of this standard, no position is taken with respect to the existence or validity of any claim or of any patent rights in connection therewith. CDISC, including the CDISC Board of Directors, shall not be responsible for identifying patent claims for which a license may be required in order to implement this standard or for conducting inquiries into the legal validity or scope of those patents or patent claims that are brought to its attention.
Representations and Warranties
"CDISC grants open public use of this User Guide (or Final Standards) under CDISC's copyright."
Each Participant in the development of this standard shall be deemed to represent, warrant, and covenant, at the time of a Contribution by such Participant (or by its Representative), that to the best of its knowledge and ability: (a) it holds or has the right to grant all relevant licenses to any of its Contributions in all jurisdictions or territories in which it holds relevant intellectual property rights; (b) there are no limits to the Participant's ability to make the grants, acknowledgments, and agreements herein; and (c) the Contribution does not subject any Contribution, Draft Standard, Final Standard, or implementations thereof, in whole or in part, to licensing obligations with additional restrictions or requirements inconsistent with those set forth in this Policy, or that would require any such Contribution, Final Standard, or implementation, in whole or in part, to be either: (i) disclosed or distributed in source code form; (ii) licensed for the purpose of making derivative works (other than as set forth in Section 4.2 of the CDISC Intellectual Property Policy ("the Policy")); or (iii) distributed at no charge, except as set forth in Sections 3, 5.1, and 4.2 of the Policy. If a Participant has knowledge that a Contribution made by any Participant or any other party may subject any Contribution, Draft Standard, Final Standard, or implementation, in whole or in part, to one or more of the licensing obligations listed in Section 9.3, such Participant shall give prompt notice of the same to the CDISC President who shall promptly notify all Participants.
No Other Warranties/Disclaimers. ALL PARTICIPANTS ACKNOWLEDGE THAT, EXCEPT AS PROVIDED UNDER SECTION 9.3 OF THE CDISC INTELLECTUAL PROPERTY POLICY, ALL DRAFT STANDARDS AND FINAL STANDARDS, AND ALL CONTRIBUTIONS TO FINAL STANDARDS AND DRAFT STANDARDS, ARE PROVIDED "AS IS" WITH NO WARRANTIES WHATSOEVER, WHETHER EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE, AND THE PARTICIPANTS, REPRESENTATIVES, THE CDISC PRESIDENT, THE CDISC BOARD OF DIRECTORS, AND CDISC EXPRESSLY DISCLAIM ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, FITNESS FOR ANY PARTICULAR OR INTENDED PURPOSE, OR ANY OTHER WARRANTY OTHERWISE ARISING OUT OF ANY PROPOSAL, FINAL STANDARDS OR DRAFT STANDARDS, OR CONTRIBUTION.
Limitation of Liability
IN NO EVENT WILL CDISC OR ANY OF ITS CONSTITUENT PARTS (INCLUDING, BUT NOT LIMITED TO, THE CDISC BOARD OF DIRECTORS, THE CDISC PRESIDENT, CDISC STAFF, AND CDISC MEMBERS) BE LIABLE TO ANY OTHER PERSON OR ENTITY FOR ANY LOSS OF PROFITS, LOSS OF USE, DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR SPECIAL DAMAGES, WHETHER UNDER CONTRACT, TORT, WARRANTY, OR OTHERWISE, ARISING IN ANY WAY OUT OF THIS POLICY OR ANY RELATED AGREEMENT, WHETHER OR NOT SUCH PARTY HAD ADVANCE NOTICE OF THE POSSIBILITY OF SUCH DAMAGES.
Note: The CDISC Intellectual Property Policy can be found at: cdisc_policy_003_intellectual_property_v201408.pdf.