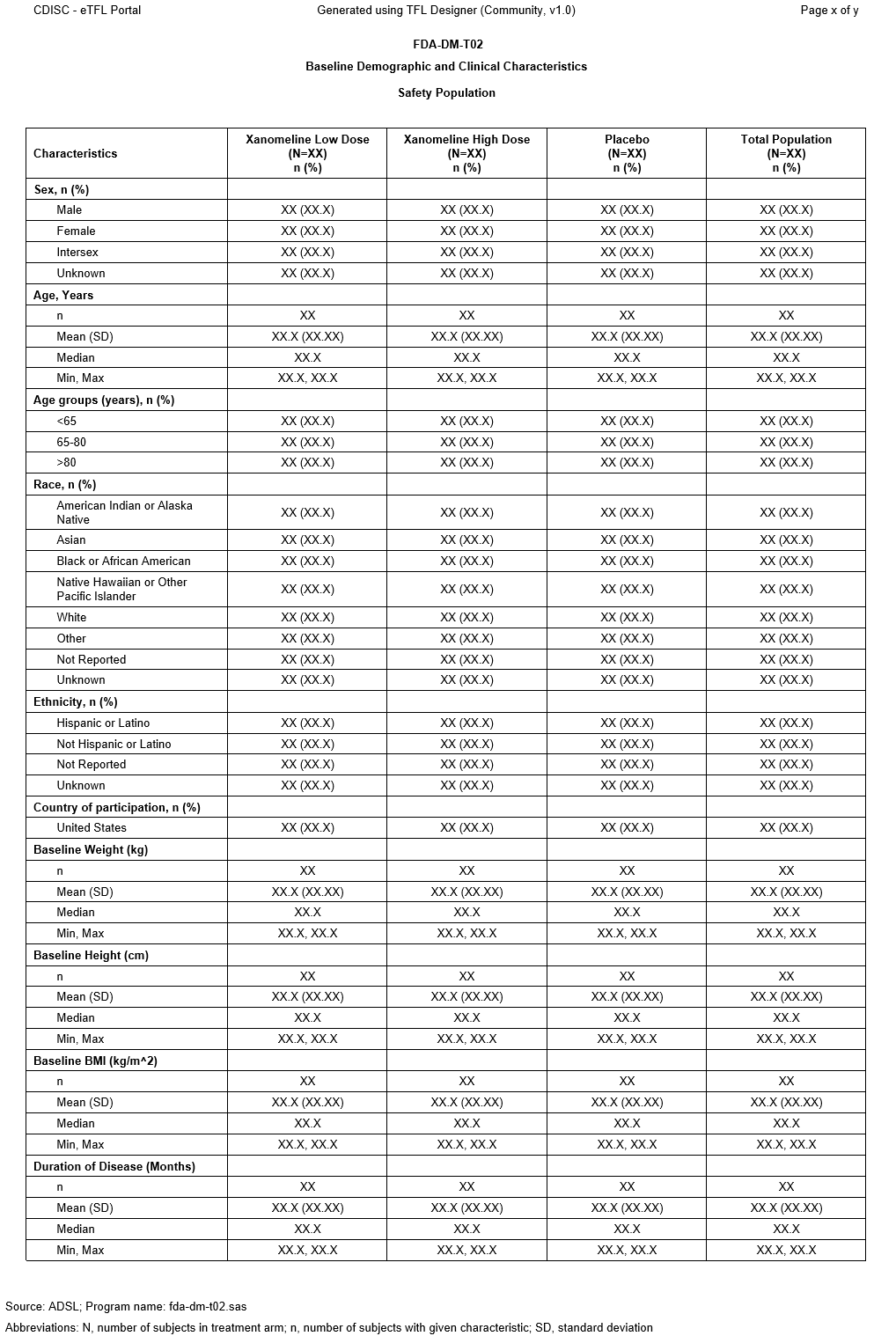
This table shows key baseline characteristics of the safety population that could influence the effectiveness or safety of the drug.
This display is based on Table 2. Baseline Demographic and Clinical Characteristics, Safety Population, Pooled Analyses (or Trial X) from the FDA STANDARD SAFETY TABLES AND FIGURES: INTEGRATED GUIDE (Version Date: August 2022), published by the Center for Drug Evaluation and Research (CDER) Biomedical Informatics and Regulatory Review Science (BIRRS) Team.
This display was created using data from the following ADaM datasets
ADSL
The ADaM datasets from the CDISC Pilot Study were modified as follows
Only variables that were needed to create this display have been retained.
If needed, additional variables were added to support the creation of the display.
The following differences exist between the display shown and the reference display from the FDA Standard Tables and Figures: Integrated Guide
The display shown uses the word "Subject" vs "Patient" to be consistent with the language used in CDISC standards.
The Active Control column was not included because there was no active control for the study.
- Baseline Weight, Baseline Height, Baseline BMI, and Duration of Disease were included as examples of baseline characteristics.
FDA-DM-T02 eTFL Package